Update:
EPM in Horses
What we know about this equine neurologic disease and where the research is headed
By Marcia King
Sponsored by:

CREDIT: The Horse | Stephanie L. Church

Suddenly, this careful, athletic horse seemed unbalanced and unsure, even just walking around the paddock. And while he was still eager to greet barn visitors and accept carrots, all it took was a swing of the neck the wrong way and his hindquarters would teeter and sway.
Equine protozoal myeloencephalitis can manifest in myriad ways, from the weakness and incoordination Icy showed to facial paralysis and difficulty swallowing. Anyone who’s managed a horse struggling with EPM knows firsthand how agonizing it can be. For instance, even after treatment Icy’s balance was never quite right again.
Fortunately, researchers and veterinarians are committed to learning all they can about the known causative organisms of EPM—the single-celled protozoan parasites Sarcocystis neurona and Neospora hughesi—how they affect the horse, and how the horse’s body responds, so they can more accurately diagnose, treat, and perhaps even one day prevent the disease. More specifically, they’re looking at why most U.S. horses have been exposed, yet just a fraction of these animals develop neurologic signs. They’re also examining how coinfections with different pathogens (disease-causing organisms)—from other protozoa to intestinal parasites—might influence disease.
Read on to learn about why EPM remains an important threat to horses, what we’re learning through research, and how we as horse owners can help.
The EPM Society endeavors to contribute to a greater understanding of equine protozoal myeloencephalitis, encourage cooperative EPM research, promote awareness of current EPM research and new developments in the field, and evaluate and disseminate guidelines for the diagnosis, treatment, and prevention of EPM.
The Parasite and the ’Possum
PHOTO: iStock
Veterinarians first described what’s now called EPM in 1968, and in the 1970s researchers identified protozoa as the cause of this devastating neurologic illness. They didn’t pinpoint S. neurona as the specific culprit until the 1990s.
Both S. neurona and N. hughesi are members of the phylum Apicomplexa, a very broad group of important pathogens found worldwide; among its ranks are the protozoa that cause malaria (a mosquito-transmitted disease affecting humans) and toxoplasmosis (a zoonotic disease notorious for putting human pregnancies at risk for premature birth and birth defects, most commonly when pregnant women are exposed to infected cat feces).
A simple way to wrap your mind around a microscopic disease-causing parasite is to remember its end game: survival. So, in most cases a parasite’s goal is not to kill its host and, thus, destroy its house—yet they can do a lot of damage to its foundation.
“The Apicomplexa are all obligate intracellular parasites,” says Dr. Dan Howe. “They have to be inside a host cell to survive.
Now think back to high school biology: “Just like us, they’ve got things like a cytoskeleton, a nucleus, endoplasmic reticulum–we share a lot of the same metabolic pathways,” adds Howe. “However, these organisms also have evolved what I like to consider a toolkit of virulence factors that allow them to do what they need to do to survive as intracellular pathogens.”
They must reproduce and, to do this, must be transmitted from one host to another. We’ll talk about S. neurona here because that’s the EPM-causing pathogen researchers know best. It is a two-host parasite: In its definitive host it undergoes sexual reproduction, in which infective structures called oocysts or sporocysts are formed in the gut and passed in the feces. In one of several possible intermediate hosts, it undergoes asexual reproduction and produces another stage of the parasite.
In many parts of the United States, if we haven’t seen S. neurona’s definitive host lumbering out of our barns, we’ve most certainly seen it as roadkill: the opossum.
“The opossum’s not too selective about where it defecates, so it ends up contaminating food and water supply for the intermediate hosts, which are things like skunks, raccoons, cats, armadillos,” says Howe. “And in these hosts, you get asexual reproduction, which culminates in the production of sarcocysts, which are cysts in the muscle tissue. When this animal dies, opossums are great scavengers; they eat the dead raccoon, and that completes the life cycle.”
Horses and sea mammals, such as otters, enter the life cycle through food and water contamination. And, while scientists consider sea mammals intermediate hosts because they’ve found sarcocysts in their muscles, Howe doesn’t think opossums dine on dead aquatic animals enough to contribute much to the natural history of S. neurona. But scientists recently figured out how sea mammals could be getting infected with the protozoa: through opossum-contaminated watershed from higher ground.
Dan Howe, PhD,
Dr. Howe describes S. neurona.

Dan Howe, PhD,
is a parasitologist and professor at the University of Kentucky’s Gluck Equine Research Center, in Lexington.

As for the S. neurona sporocysts that infect the horse, they are environmentally resistant. “It takes one infected opossum to go through your barn, defecating a few times, and then you’re going to have those oocysts in your barn for a while,” says Howe, “So, yes, we want to encourage owners to do what they can to prevent opossums from getting into their food and water sources in their barns, but you can’t completely prevent it, and once it’s there, that environment is seeded with those oocytes. Experiments done with Toxoplasma (again, a closely related protozoon) show they’re good (viable) for over a year or more.”
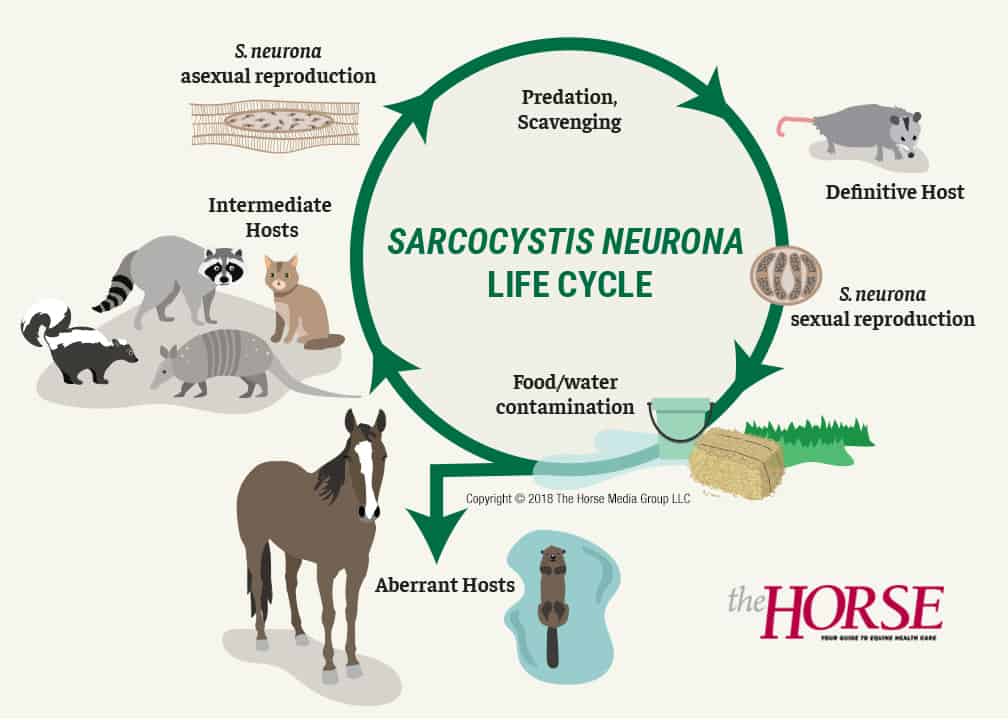
Howe emphasizes this is the fundamental mode of S. neurona transmission to the horse.
“I’d like to say it’s the only way, but at least one case suggests that you can have transplacental passage from the mare to the foal,” he says. “(It’s) probably very rare, and I think it’s likely something that occurs when the mare is acutely infected—she gets exposed while she’s pregnant and, in that case, you might get the transplacental passage,” rather than reactivation of a dormant infection.
Researchers aren’t sure exactly how S. neurona gets into the horse’s central nervous system (CNS) after he inadvertently eats sporocyst- or oocyte-containing opossum feces, but they suspect it crosses the blood-brain barrier through cell transport into the brain and spinal cord. The horse does not generally develop cysts in the muscle the way intermediate hosts do.

Nicola Pusterla,
DVM, PhD, Dipl. ACVIM,
is a parasitologist and professor at the University of Kentucky’s Gluck Equine Research Center, in Lexington.
Howe emphasizes this is the fundamental mode of S. neurona transmission to the horse.
“I’d like to say it’s the only way, but at least one case suggests that you can have transplacental passage from the mare to the foal,” he says. “(It’s) probably very rare, and I think it’s likely something that occurs when the mare is acutely infected—she gets exposed while she’s pregnant and, in that case, you might get the transplacental passage,” rather than reactivation of a dormant infection.
Researchers aren’t sure exactly how S. neurona gets into the horse’s central nervous system (CNS) after he inadvertently eats sporocyst- or oocyte-containing opossum feces, but they suspect it crosses the blood-brain barrier through cell transport into the brain and spinal cord. The horse does not generally develop cysts in the muscle the way intermediate hosts do.

Dr. Nicola Pusterla has a special research interest in EPM and says there are still some questions to be answered about the S. neurona life cycle and where horses fit. They could be accidental or immediate hosts, he explains, and even that could depend on the S. neurona strain, the horse’s environment, how intermediate and definitive hosts interact, or other factors.
“I think it’s important information if we’re wanting to look at trying to understand this disease and how it happens,” he says.
But why my horse?
PHOTO: iStock

Stephen Reed, DVM, Dipl. ACVIM,
is an internal medicine specialist and shareholder at Rood & Riddle Equine Hospital, in Lexington, Kentucky, where he focuses on equine neurologic diseases. He’s also emeritus professor of The Ohio State University, an adjunct professor at the University of Kentucky and is currently the chair of the Grayson-Jockey Club Research Foundation research advisory committee.
However infection happens, and whatever the horse’s role, animals that get EPM develop a variety of clinical signs. These can be acute or chronic, seemingly isolated to one area of the horse—based on which section of the CNS the parasite has affected—or all over.
Authors of the most recent update to the American College of Veterinary Medicine’s EPM Consensus Statement say the initial signs of EPM can include:
- Difficulty swallowing
- Abnormal airway function
- Unusual or atypical lameness (think stumbling, frequent limb interference in horses that wouldn’t generally show this)
- Seizures
Dr. Steve Reed
Dr. Reed describes clinical signs of EPM.
Dr. Stephen Reed has studied EPM for 30-plus years. “EPM can present in many ways, but some of the common presentations involve asymmetric gaits with horses showing weakness and ataxia in one or more limbs,” he says. “Often the signs are accompanied by muscle atrophy, which can be seen on the head, trunk or limbs.
“Sometimes horses present with cranial nerve deficits such as head tilt, dysphagia, or abnormal phonation (sounds),” he adds, referring to the nerves which arise from the brain and brain stem. “Thus, careful examination of all cranial nerves is important.”
Reed explains that in a recent review of medical records from the University of California, Davis, and Rood & Riddle Equine Hospital, veterinarians noted the asymmetric signs and, in another review of records from Ohio State and Rood & Riddle, veterinarians had observed a history of behavior changes in many horses confirmed to have EPM.
“Onset of signs can be quite acute,” says Reed, “but in my experience there’s usually a more insidious onset with variable rate of progression of signs (how quickly signs worsen varies from horse to horse). The important thing to remember is to be thorough on the neurologic exam, as some of the earliest signs might be intermittent dragging of the pelvic (hind) limb toes or a focal atrophy of muscles on the face or forehead.”
In severe cases horses can have trouble standing, walking, or swallowing, and their condition might deteriorate quickly, progressing to sudden recumbency (inability to rise), which can require euthanasia. (In Icy’s case his incoordination led to a fall, while he was playing with a buddy, and he was unable to rise.)
One thing veterinarians have to keep in mind when they’re examining a potential EPM case is the many causes of neurologic disease. They need to rule out these differentials.
EPM Case Example
“With spinal cord signs, cervical vertebral myeloencephalopathy, or true Wobbler (syndrome), would be the first big differential to eliminate,” says Reed. “If you have a Quarter Horse, then you might give consideration to equine degenerative myelopathy, because that’s another big spinal cord problem, and then, beyond that, trauma is always going have to be listed as a differential.
“Recently we’ve been hearing lots and lots about herpesvirus (equine herpesvirus-1, or EHV-1) myeloencephalopathy,” he adds, noting that while EHV-1 neurologic cases can look like EPM, generally more than one horse is affected with EHV-1, and often they have fevers.
But sometimes EPM cases don’t look like how veterinarians would expect. It can appear as an unusual change in behavior, which Reed says owners sometimes report, or an unusual lameness.
“Yesterday we had a horse that presented … for a lameness examination because it showed stringhalt, and that horse ended up … a very strong positive for EPM,” says Reed. “So even some of the obscure lamenesses, like stringhalt and shivers, and things like that, sometimes need to be evaluated to make certain that they don’t have EPM.”
As mentioned before, the strange thing about S. neurona is the sheer amount of horses with exposure evident in their blood serum but no clinical signs. Here’s another place where researchers are working hard.
In previous studies, researchers determined that 61.8% of EPM cases occurred in horses 4 years old or younger, and 19.8% of cases were in horses 8 years old or older. They most commonly observed EPM signs in Thoroughbreds, Standardbreds, and Quarter Horses, but with no sex or seasonal bias. The mean age of affected horses has been 3.6 ± 2.8 years. In a more recent study on proportional morbidity rate (an indicator of incidence of EPM among a population of horses), researchers found a higher incidence among Standardbreds, Tennessee Walking Horses, Thoroughbreds, Warmbloods, and stallions. Quarter Horses, other breeds, ponies, and drafts had a lower incidence rate.

Other researchers are going even deeper, looking at why horses get EPM on an individual level. In an ongoing multidisciplinary study Dr. Sharon Witonsky has been examining the immune phenotypes (the specific types of immune responses that the body makes) of horses that develop EPM clinical signs versus those that don’t.
“The factors that have been identified to be stressful (for horses, leaving them vulnerable to EPM) are shipping, showing, training, and pregnancy,” says Witonsky. “We see effects of seasonality and location, age, and other ongoing diseases.”
Specifically, she is looking at how horses respond to infection. Horses develop two types of immune responses when exposed to a pathogen: humoral and cell-mediated. The humoral immune response is systemic and involves the production of antibodies, or blood proteins the body creates in response to and counteracting specific antigens, or foreign substances. One of the things that the cell-mediated response does is protect the body against intracellular organisms using special white blood cells called T-cells (T-lymphocytes). T-cells recognize cells that have been infected before, and the goal is for the immune system to kill S. neurona.
Witonsky says researchers have data to support that horses need to develop a cell-mediated response to protect against S. neurona.
“For the predicted protective immune response … the question is really what happens with the horses’ disease,” says Witonsky. “We just don’t know, do the horses that we’re putting down for EPM or that get severe clinical signs, do they not make enough of a particular cell-mediated response, or is production of an inflammatory cytokine (another type of protein that affects cell interaction and communication) preventing it?
“I do think we have some interesting preliminary data, and I’m hoping to build on that.”
Reed adds: “This is an open window now, is looking at and understanding of the role of what is a normal immune response compared to horses that exhibit an abnormal immune response. Because since many, many horses are exposed to the organism, and mount antibodies, that means a majority of horses get the organism under control. It’s the ones that seem to be either immunosuppressed or that generate an improper immune response that develop neurologic illness.”
Dr. Sharon Witonsky
Dr. Witonsky describes immune responses in EPM.

Sharon Witonsky,
DVM, PhD, Dipl. ACVIM,
is associate professor of Equine Field Service in the Department of Large Animal Clinical Sciences at the Virginia-Maryland Regional College of Veterinary Medicine, in Blacksburg, Virginia.
How do Veterinarians know it's EPM?
PHOTO: Alexandra Beckstett
For many years veterinarians used a test called the Western blot as the standard for diagnosing EPM in the live horse. It worked by detecting a pattern of S. neurona antibodies in the horse’s serum or cerebrospinal fluid (CSF). Tests have evolved dramatically in the past decade, and authors described them in detail in the ACVIM’s revised consensus statement.
“You have a document that is based on all scientific material and gives you good guidelines,” says Pusterla. “It reviewed a lot of areas, but most of the areas that I thought as a clinician were very important were redefining how we get to a diagnosis.”
The authors defined the gold standard for diagnosing EPM in the living horse:
- Observable neurologic signs consistent with EPM
- A positive serum:CSF ratio, which indicates intrathecal (within the central nervous system) antibody production against S. neurona or N. hughesi
- Ruling out diseases with similar neurologic signs
Dr. Amy Johnson
Dr. Johnson describes EPM diagnostics.
But Pusterla says some veterinarians avoid collecting CSF because the procedure can be challenging for the veterinarian and because it’s possible to contaminate the sample with blood on collection. Or, the owner simply might not want to pursue it due to cost or their perception of the procedure.
Some veterinarians can be reluctant to collect CSF, says Pusterla, but “the reality is without that CSF, we can’t confidently rule in or rule out EPM.”
In the meantime, practitioners have developed and presented an alternative way of using ultrasound-guided CSF collection, via the cervical spine, that they claim is safer for veterinarians and potentially easier for them to learn.
The currently favored tests used on serum and CSF work by measuring antibodies to proteins on the outer surface of different strains of S. neurona that trigger the horse’s immune system. The specific tests the consensus statement authors recommended are based on six test comparison studies, none of which compares all of the four commercially available antibody tests to each other or using the same samples, explains Dr. Jennifer Morrow. It should be noted that the four tests differ in how they work and, most importantly, how or if they were validated. She says the serum tests are less accurate, due to low specificity (they’re likely to produce false positives), hence, the recommendation for CSF confirmation and, ideally, a serum:CSF ratio.

Amy Johnson, DVM, Dipl. ACVIM (large animal internal medicine, neurology),
is an assistant professor of large animal medicine and neurology in the University of Pennsylvania School of Veterinary Medicine’s New Bolton Center, in Kennett Square.
| Test | Looks For | Used On | Summary of Conclusions/Comparisons |
| Western blot (WB) | Patterns of S. neurona antibodies | Serum and cerebrospinal fluid (CSF) | |
| Indirect fluorescence antibody test (IFAT) | Antibodies against the whole parasite | Serum and CSF | IFAT accuracy was better than WB tests |
| SAG2, 4/3 ELISA (antibodies against surface antigens detected with enzyme-linked immunosorbent assay) | Antibodies to three separate proteins on the outer surface of S. neurona; these three proteins are well-conserved across strains | Serum and CSF | Ratio is most accurate in all studies that assessed it (three of six studies, in which it was compared to WB, SAG1—which is no longer available, and IFAT) |
| SAG1 5,6 ELISA | Antibodies to three separate proteins on the outer surface of different S. neurona strains | Serum and CSF | No comparison studies available |
Three Recommendations for Diagnosing EPM
- Neurologic exam to confirm clinical signs
- Exclusion of other diseases that could look like EPM
- Antibody testing of serum and cerebrospinal fluid (confirming intrathecal—inside the central nervous system—antibody production)
Still, to this day, the only way to definitively diagnose EPM in horses is finding the protozoa in the CNS on a post-mortem examination. Interestingly, pathologists have found that the acute cases—horses that are euthanized during their first visible signs of EPM—have more S. neurona in the tissues, whereas chronically affected EPM horses have more signs of damage across different components of the nervous system but fewer S. neurona present. The lack of visible parasites in chronic cases likely is due to drug treatment and immune clearance of the parasites, says Johnson. So for post-mortem diagnosis, pathologists see more of the pathogen in the acute cases.
Some veterinarians rely on how a horse responds to a treatment trial to seal their diagnosis. But researchers haven’t defined how quickly or how much the horse must improve for it to be considered an EPM case. Pusterla says when he uses a treatment trial, if the horse hasn’t made a 25% improvement within three weeks, he revisits whether it’s EPM or another neurologic disease.
As for future directions in testing, “clinicians want reliable, cost-effective, and properly validated diagnostics,” says Pusterla. Researchers have tried measuring a few proteins the horse’s liver releases in response to acute inflammation, called acute phase proteins. They found that serum amyloid A (SAA) and C-reactive protein (CRP)—two proteins known to rise in the face of other types of infection-caused inflammation in horses–were not useful biomarkers for confirming EPM infection.
Treatment Options
PHOTO: The Horse Staff
Veterinarians have at their disposal three FDA-approved products for treating EPM:
- Sulfadiazine/pyrimethamine (ReBalance)
- Ponazuril (Marquis)
- Diclazuril (Protazil)
These products are labeled for 28 days of treatment, though how long horses need to stay on the drugs usually depends on their treatment response. Consensus statement authors say most horses with EPM are treated for six to eight weeks or longer, if clinical improvement is still apparent under treatment. Studies have shown that 60% of the drug-approval field trial horses showed improvement.
“There was never and has never been, to my knowledge, a rationale for treatment durations that we use in these drugs,” says Dr. Rob MacKay, who’s devoted much of his career to EPM research. “Horses were enrolled (in the original drug trials performed around 20 years ago) on clinical signs plus Western blot (test), so … that’s likely led to an underestimate of treatment efficacy, because we presume we enrolled some ‘false-positive’ horses that didn’t have EPM.”
Pusterla agrees. “Today, with quantitative immunodiagnostics (and a) more standardized neuro exam, if we had to repeat these studies, I believe the odds (of success) would be so much better out there. I see the success based on these drugs, so I’m going to continue to use them.”
Researchers haven’t compared the efficacy of these drugs to one another in large, case-control studies because having a control (untreated) group cannot happen in a patient population. They would need to run it in a research population, which would be both time-consuming and very expensive.
Dr. Steve Reed
Dr. Reed describes EPM treatment options.
There are several unofficial, off-label, and illegal drugs people use to treat EPM:
- Toltrazuril (Baycox, which is not approved in the U.S.)
- Compounded drugs (toltrazuril ± pyrimethamine, sulfadiazine/pyrimethamine—the latter is illegal because an FDA-approved version is available)
- Diclazuril (again, illegal because an FDA-approved version is available) used as a sodium salt or intravenously
“There is a massive ‘gray market’ of Baycox (toltrazuril) imported from Australia and/or Canada and huge amounts of Baycox used on young racehorses that are for various reasons, including suspect EPM, but not limited to suspect EPM,” says MacKay. “Bit of an outrage, I’d say.
“Compounded sulfadiazine/pyrimethamine shouldn’t be happening, and we should discourage it by prescribing (the FDA-approved product),” he says. On one occasion a compounder reversed the concentrations, which caused toxicity in five horses.
A “very promising” EPM drug is in the FDA pipeline, says MacKay. The drug, decoquinate, which is sometimes used in combination with the immunostimulant levamisole, could reach market within a few years.
“A new field that is expanding is prevention,” says Pusterla. “Vets, clients, owners don’t want to risk losing a horse to EPM.” That has led some racehorse trainers to give these treatments to valuable racehorses preventively. The problem is, veterinarians don’t know what constitutes a protected horse—they haven’t challenged horses on low doses of the drugs with S. neurona to see if it’s effective.
“We haven’t tested 200 to 300 horses, knowing, ‘Hey, that’s a protocol that really works,’” says Pusterla.
In the early 2000s an EPM vaccine was briefly available, but production stopped because it was difficult to prove the vaccine’s efficacy in the existing research model, which involves challenging horses with protozoa produced by families of opossums and raccoons in the laboratory. Pusterla explains that if a vaccination were available now, it could complicate serum and CSF testing. Why so? When vaccinating a horse, you stimulate his immune system to produce antibodies against a pathogen, so it can fight it if ever exposed. Higher antibody levels against S. neurona–besides the ones from natural exposure, which tests already account for–could just confuse results, making it impossible to distinguish an infected horse from a vaccinated one.

Dr. Rob MacKay, BVSc, PhD, Dipl. ACVIM,
is a professor at the University of Florida, in Gainesville. His interests include internal medicine and clinical neurology.
N. Hughesi: The Often Overlooked EPM Parasite
PHOTO: Dr. J.P. Dubey
“There’s a lot known about the biology, the life cycle for Sarcocystis neurona, but not so much for Neospora hughesi, the diagnostics, or what they are,” says Pusterla.
“That’s the neglected middle child of the EPM world,” says Pusterla, “but at least we’ve made a little improvement there showing that Neospora hughesi infection do actually occur outside California. We’re moving in the right direction.”
Things researchers do know about N. hughesi:
- Vertical transmission can happen from the mare to the developing foal
- It also likely has a two-host life cycle
The horse (among other animals) can also serve as an intermediate host

“Because of what we know with Neospora caninum (a closely related organism),” says Howe, “I think it’s possible to say that canines are the definitive host for Neospora hughesi as well.”
Howe and other researchers also compare N. hughesi to the parasite T. gondii, because in its intermediate hosts it recrudesces, or reactivates, from a slow-growing stage.
He explains that these N. hughesi cysts can reverse to the fast-growing stage and, because a mare can transmit the pathogen to her foal via placenta, he thinks cyst recrudescence can happen there, too.
With Sarcocystis, however, all the evidence indicates that recrudescence cannot occur because it has gone down a developmental path to the point where it can’t turn around but only develop further in the gut of the opossum.
When Multiple Parasites Move In
An area in which researchers are especially interested is a concept called comorbidity—or illness caused by a combination of parasites. To understand how two parasites cohabitate and even help each other, it’s important to understand how they work on their own.
Dr. Martin Furr
Dr. Furr describes comorbidity.
“In general, when we think about parasitic infections in immunocompetent hosts, how do they survive?” says Dr. Martin Furr, head of the Department of Physiological Sciences at Oklahoma State University’s Center for Veterinary Health Sciences. “Well, they employ a variety of mechanisms. One is they outrun the host response by fast replication or some form of mutation. Or, they impair the development and expression of a specific or innate immunity from the host through some version of immune evasion or immune modulation or restriction.”
In other words, they somehow hamper the horse’s ability to mount an immune response. He explains that the result of that process is a “blunted, nonsterilizing immunity” (in the horse), which reduces the number of parasites and limits the damage they can cause, but without completely eliminating or wiping them out, Furr adds. Clearly, the immune system and modulation of the immune system responses are very important.
With coinfections, the thought is that when a non-EPM infection causes an immune response but then downplays it to survive—so as not to kill its “house”—it might create a vulnerable environment for a secondary infection by S. neurona or N. hughesi to come along. Yet, even with two infections, it doesn’t necessarily mean the clinical signs are worse.
“The idea that you get infected with one parasite and then you get infected with a second parasite and the pathology is worse and worse, the more organisms are there, doesn’t seem to be true,” says Furr. “In fact, they seem to support each other, and it’s very interesting that the protection is stage-specific (only during certain parts of the parasite life cycle), and there appears to be a symbiosis almost between the parasites and the host.
“Polyparasitism is probably more common that we realize,” says Furr, and in veterinary species, it’s not only parasitic infections but also bacterial.
When it does happen, which for the combination of S. neurona and N. hughesi is fairly rare (0.8% of EPM suspect cases—and even more rare in the Eastern United States, where vets haven’t seen any co-infections of N. hughesi or T. gondii in EPM cases), “is Sarcocystis really inducing an immunosuppressive environment that’s allowing secondary infections to occur?” poses Furr. “Or is it responding, as an opportunist, to an immunosuppressive event maybe caused by another organism? Another parasite, N. hughesi perhaps, intestinal parasitism, herpesvirus, something else?”

Martin Furr,
DVM, Dipl. ACVIM, PhD, MA Ed,
is the head of the Department of Physiological Sciences at Oklahoma State University’s Center for Veterinary Health Sciences.
Future Directions
PHOTO: iStock
We’ve come a long way in those 50 years since researchers first identified EPM. Yet scientists continue to charge forward in their research areas, contributing to the body of knowledge that guides them in their diagnostic and treatment decisions. Here are some areas Pusterla expects or hopes to see growth and generate new information:
Reference Sample Library Pusterla hopes scientists can work together to build a repository of samples from both normal horses and those with confirmed EPM. This would allow them to screen and validate new diagnostic tests and more confidently diagnose cases and prescribe treatment.
“Even if a horse walks and quacks like it has EPM, we still get fooled” sometimes, says Pusterla. “There is no way to build ego with EPM.”
Dr. Nicola Pusterla
Dr. Pusterla sums up research progress and challenges associated with understanding EPM.
CSF Taps vs. Treatment Trials He drives home the importance of CSF collection for diagnostic consideration and questions the value of treating the horse in lieu of it and waiting for a response, noting that a cost-analysis of a treatment trial versus CSF collection could be valuable.
Comparative Studies and Polyparasitism Key to correct diagnosis and treatment is understanding the parasites at play. And by looking at closely related pathogens and studying their pathways of inflammation, scientists can translate the findings to S. neurona and N. hughesi. And then there’s the comorbidity question and its importance.
Treatment, Future Preventive Approaches Finally, there’s treatment and prevention. “Although we have good products out there, I think it’s worthwhile looking at the modeling of these products and figuring out what are minimal doses, how can we space (them) to make it cost-effective to treat these horses with safe FDA-approved drugs.” (We don’t have a long-term metric at efficacy of treatment.)
Reed sees a lot of potential for the future of EPM diagnostics—specifically, with next-generation genetic sequencing in a research area called metabolomics. This involves figuring out the unique chemical fingerprints that specific processes leave behind. The specific process here would be EPM infection. “If, in fact, we could identify some sort of a metabolomic marker that was very accurate in blood alone, then that would be great, if we didn’t have to do spinal taps,” says Reed. “We’re a ways down the road from that, but I do think that looking at that metabolomic and other next-generation sequencing is going to be helpful for diagnostics.
“There are new patterns of diagnostic testing that are going to come on the horizon,” he adds, “and it might be 10 years away … but they’re becoming more and more common in people diseases and much more common in veterinary diseases, and there’s lots of companies developing trying to develop what we can do on the veterinary side.”
It’s too late for researchers’ latest findings to make a difference in Icy’s condition, but these discoveries, these steps toward understanding EPM infection, could help countless other owners manage their animals for return to performance and quality of life. And we can all support researchers through donations and spreading the word about their efforts.
If you have managed or are managing a horse with EPM, tweet, Instagram or post on Facebook a photo of him or her with the hashtag #BeatingEPM and why you feel EPM research is important.
EPM Society
A group of dedicated equine researchers and clinicians determined to beat EPM
In 2001 scientists with an interest in equine protozoal myeloencephalitis (EPM) formed the EPM Society. Made up of researchers and clinicians from veterinary schools, private practice, and industry around the United States and world, the group convenes every three to four years to brainstorm current approaches and new directions to managing the disease; they try to make sense of the complex causative parasites Sarcocystis neurona and Neospora hughesi, how and why they infect horses, and how we can best manage cases.
“It’s fascinating how the industry works, and that’s true for most infectious diseases,” says Dr. Nicola Pusterla. “The (new) disease comes, the disease is characterized, we’ve got clinical presentation we recognize, we start evolving tests, we get to the treatment, and … that’s it.”
He says scientists often don’t progress beyond that because of a lack of funding, so they move on to the next disease. “And we realize that although we know a lot about it,” he says, “there are a lot of questions still out there.”
The society is making EPM the exception to that rule, keeping a dialogue going even decades after treatments started coming on the market. An example of their work’s tangible results can be seen in the current American College of Veterinary Internal Medicine’s EPM Consensus statement, which they revised a few years ago. Among other things, this document defines the proper steps for testing, as the disease can be very difficult to diagnose in the live horse.
The past two society meetings show the breadth of interest and progress in this research area: In 2014 researchers presented six abstracts—in 2017 they presented 22.
“If we can at least come together and move in the right direction and help … that’s the beauty here, we have basic researchers, we have diagnosticians, we have immunologists, we have clinicians, we have a variety of people with different interests and different experiences, and that’s the hallmark … to brainstorm and really move the field and figure out what needs to be done.” –Stephanie L. Church
Stephanie L. Church is editor-in-chief of The Horse: Your Guide To Equine Health Care/TheHorse.com. A 4-H and United States Pony Club (USPC) member, she grew up riding hunters and then eventing. She earned a B.A. in Journalism and Equestrian Studies from Averett University in Danville, Virginia, and joined The Horse team in 1999. She is a past-president of American Horse Publications and volunteers with the Lexington (Kentucky) Mounted Police Unit. Currently she trains her retired racehorse, It Happened Again, in dressage and eventing. She enjoys learning and writing about equine disease and epidemiology; her all-time favorite mount, retired racehorse Icy Edge, died due to complications from EPM at age 30.
Editor-in-Chief: Stephanie L. Church
Digital Editor: Michelle Anderson
Editorial Team: Alexandra Beckstett, Erica Larson
Illustrator: Claudia Summers
Art Director: Brian Turner
Digital Producer: Jennifer Whittle
Publisher: Marla Bickel







