Imagine a foreign disease, not so long ago making its way steadily across the country, killing horses one mosquito bite at a time. This disease wasn’t easily spotted; after all, horses weren’t dropping as they’re bitten. Rather, development of the illness was an insidious, slow process that had you wondering what’s wrong with your horse for hours or maybe even days. The horse acted a little depressed, then eventually started to tremble and twitch. Then he couldn’t get up. And, finally, with the help of your veterinarian, you were forced to say goodbye.
West Nile Virus
A mosquito-borne zoonotic (affecting humans and specific animal species) arbovirus belonging to the genus Flavivirus in the family Flaviviridae. It’s commonly found in temperate and tropical regions of the world. Horses are highly susceptible to the virus, which causes the neurologic disease West Nile encephalitis.
AAEP Core Vaccines
Eastern/Western equine encephalitis, Rabies, Tetanus, and West Nile virus
Today, West Nile virus (WNV) might seem like just another preventable disease and a shot your horse gets once or twice a year—a single vaccine listed among several others in the American Association of Equine Practitioners’ (AAEP) core recommendations. But, nearly 24 years ago, this virus and the severe neurologic disease it causes—which affects many species, including humans —posed the biggest health threat the horse industry had seen in the 20th century. This is the story of WNV in North America. It’s a story of a real risk to our country’s horses, as well as rapid spread of fear and irrational rumors. It’s also a story about how the veterinary community, U.S. government, and animal health industry joined forces to find a way to protect our horses.
WNV Lands in the United States
Physicians first identified WNV in 1937 in a female resident of Uganda, Africa’s West Nile district. After that, the virus caused several outbreaks in the Eastern Hemisphere and was first identified in horses in 1960. Today, WNV is the most widely distributed of the arboviruses (viruses spread by arthropods such as a ticks or mosquitoes), causing infections in Africa, the Middle East, South Asia, Australia, Southern Europe, and North and South America.
But until 1999, few American horse owners had heard of the virus.
That changed late in summer ’99, when authorities confirmed the first WNV case in the Western Hemisphere in Queens, New York. They first identified it in birds, and three months later confirmed the first human case. Doctors and veterinarians subsequently reported cases of WNV-related fever and encephalitis in humans, birds, and horses, and the virus started its march into New Jersey and Connecticut. The veterinary community and horse industry went on alert—the disease was aggressive in horses, and at the time we had no vaccine to protect them or a way to provide specific treatment for the virus.
Luckily, experts already understood WNV’s basic life cycle from intelligence data collected by U.S. researchers stationed in Egypt during the 1950s. Specifically, they knew the cycle relied heavily on birds and mosquitoes.





But how did this virus get from Africa, the Middle East, and Europe to the United States in the first place? An airplane takes about 24 hours to travel the almost 7,900 miles from Uganda to New York. The trip requires Atlantic Ocean and Mediterranean Sea crossings and traversing three continents, with a layover in Europe.
“There are actually about 13 viable theories of how the WNV got to New York City” says Nicholas Komar, SD, a biologist with the Centers for Disease Control (CDC), in Fort Collins, Colorado. “My favorite (theory) is that an infected mosquito came over in an airplane, deplaned in New York City, and fed on an urban bird like a house sparrow, initiating a local amplification among the large population of bird-feeding Culex pipiens (mosquito species), which thrived in New York City in 1999 due to local drought conditions and absence of mosquito control operations.”










Other potential routes of introduction include sources of infected birds or mosquitoes, especially considering the close proximity of two international airports—JFK and LaGuardia—as well as the Bronx Zoo near the disease’s initial point of diagnosis, where several bird residents had died of WNV starting over Labor Day weekend. Those early avian deaths included a pheasant, bald eagle, cormorant, and flamingos. Experts have said it was unlikely foreign birds legally imported to the Bronx Zoo or resident zoo birds were sources of infection because wild birds, including crows, outside the zoo had died first.
Nicholas Komar, SD,
serves in the Arbovirus Diseases Branch of the Centers for Disease Control’s National Center for Emerging Zoonotic Infection Diseases Division of Vector-Borne Diseases. He’s a WNV expert who’s published numerous scientific papers on the disease.
Viremia:
Viral Load
A WESTWARD EXPANSION
Much to the chagrin of researchers and veterinarians, albeit not to their surprise, the virus re-emerged when the weather warmed in early 2000 and mosquitoes started breeding.
“I used to think all mosquitoes were created equal but I learned (from WNV) that they have a preference for breeding grounds and what animal they feed off,” says Dr. Josie Traub-Dargatz of Colorado State University. “It is the lack of focused feeding of certain types of mosquitoes that contributed to the spread of WNV.”
While the details of the virus’ journey to New York remain a mystery, some evidence suggests it might have originated in Israel. Genetic analysis completed within four short weeks of the outbreak’s start revealed that the New York virus most closely matched the one responsible for a 1998 outbreak in Israeli birds, including geese. The virus identified in New York also was similar to WNV found in Israeli patients in 1999. This strain caused a higher rate of avian deaths than previously studied WNV strains.
“Because the virus was a more virulent avian strain, more birds had higher viremia, making them capable of infecting more mosquitoes,” Komar explains. “In turn, this would tangentially infect more susceptible dead-end hosts, such as humans and horses.” (Dead-end hosts cannot serve as reservoirs for the virus and, therefore, other individuals cannot contract the disease directly from them.)
This didn’t bode well for either species stateside.
By year’s end 1999, authorities had confirmed 25 equine WNV cases, all in New York. Once U.S. health authorities correctly identified WNV that year, some experts held out hope that the cold winter months would kill infected mosquitoes and, in turn, eliminate the virus. But, alas, the virus had already spread, with infected mosquitoes and birds quickly identified in several more temperate states.
Epidemiologists and health authorities believed that the New York subway system environment, as well as storm-water drainage systems and basements, allowed infected mosquitoes to overwinter and serve as a source of infection in 2000. They further speculated that adult mosquitoes could pass on the virus to their offspring, a process called “transovarial transmission.”
Despite the widespread implementation of mosquito-control strategies (e.g., spraying of pesticides, limiting breeding-ground-providing standing water) WNV continued to flourish, largely because it did not behave like other arboviruses already present in the United States, which have geographic niches they rarely stray from: Eastern equine encephalitis (EEE) virus generally stays in the East and Western equine encephalitis (WEE) virus in the West. WNV, however, spread all directions.
A steady climb in equine cases in the Northeast ensued.
In 2001, the virus moved rapidly north to south, which experts expected due to migration routes of birds carrying the virus. The virus’ rapid east-to-west progression, however, surprised researchers. The disease reached California by 2003, less than four years after its North American introduction.

Josie Traub-Dargatz, DVM, MS, Dipl. ACVIM,
is a professor emeritus at Colorado State University’s College of Veterinary Medicine and Biomedical Sciences in Fort Collins.
U.S. Equine WNV Cases 1999-2022
Press the play button to automatically advance through the years. Click on a year in the bar graph to see the map for that year. Hover over a state to see the number of cases reported in that state. Data source: CDC
- 0 cases
- 1-24 cases
- 25-199 cases
- 200-399 cases
- 400-799 cases
- 800+ cases
Total U.S. WNV Cases
- 1999 25
- 2000 60
- 2001 738
- 2002 15,257
- 2003 5,181
- 2004 1,406
- 2005 1,088
- 2006 1,086
- 2007 468
- 2008 179
- 2009 276
- 2010 125
- 2011 87
- 2012 627
- 2013 377
- 2014 141
- 2015 225
- 2016 380
- 2017 307
- 2018 494
- 2019 89
- 2020 71
- 2021 220
- 2022 116
Experts suggested that the virus accomplished this by “hitching a ride” via another specific type of mosquito species to the western United States.
“Once the virus crossed the Mississippi in 2002, its westward spread was certainly aided by the abundant WNV vector, Culex tarsalis, however, significant jumps from the Mississippi to a region far enough west where C. tarsalis are located could have been aided by human-built transport vessels like cars, trucks, trains, and planes,” Komar explains. “C. tarsalis is only present in the West and they are not known to fly long distances.”
Although surprised at the relentless spread of the virus, experts did not stand idly by waiting to see how much worse each year would get for horses and owners.
PROTECTING HORSES WITH A CONDITIONAL VACCINE
As the virus spread in those early days, Fort Dodge (now Zoetis) researchers worked to concoct the first equine WNV vaccine. By 2001—within two years of identifying the first cases in New York—USDA’s Animal and Plant Health Inspection Service (APHIS) issued a “conditional license” for the vaccine, branded as “Innovator.”
“Data required for a conditional license are reduced from that needed for a full license in that the (manufacturer) needs to demonstrate a ‘reasonable expectation of efficacy’ as defined by USDA,” explained Dr. Mary Beth Evans, an APHIS licensing reviewer, who spoke with TheHorse.com in 2015 prior to her passing in 2022. “Conditional products must meet the same safety and purity requirements as fully licensed products. That the vaccine was manufactured as an inactivated product (produced using killed virus) and the chemical components used had previously been shown to be safe, the risk of a catastrophic safety issue was a minuscule event.”
“There are few who would argue that the rapid development of the vaccine didn’t play a key role in the control of WNV in those early years.”
Dr. Josie Traub-Dargatz
The West Nile virus vaccine ultimately received a full license in 2003. Data collected on the efficacy of the vaccine showed that 95% of horses that received two doses and were experimentally challenged with the virus one year later didn’t exhibit viremia, while 82% of similarly challenged unvaccinated horses developed active WNV infection.
“That product became one of the most popular vaccines in the United States, and I think it is amazing that the industry accepted and implemented it so quickly, recognizing that the term ‘conditional’ (could have been viewed by the public as) synonymous with ‘not safe,’” Traub-Dargatz says. “There are few who would argue that the rapid development of the vaccine didn’t play a key role in the control of WNV in those early years.”
She adds that numbers peaked due to WNV’s spread and “the fact that the U.S. horses were naive and there was no vaccine until 2001 and some owners were reluctant to have their horses vaccinated due to cost and the fact the vaccine was only conditionally licensed to begin with.
“Even with the availability of WNV vaccines for horses, the number of equine cases does vary year to year depending on mosquito vector numbers and, perhaps, with what percent of the equine population is current on their WNV vaccination,” says Traub-Dargatz.
Mary Beth Evans, DVM, MS,
had a long and successful career at USDA APHIS National Veterinary Services Laboratory in Ames, Iowa, and was a senior staff veterinary medical officer with the APHIS at the time of her interview. Evans, an accomplished equestrian and animal lover, died in 2022.
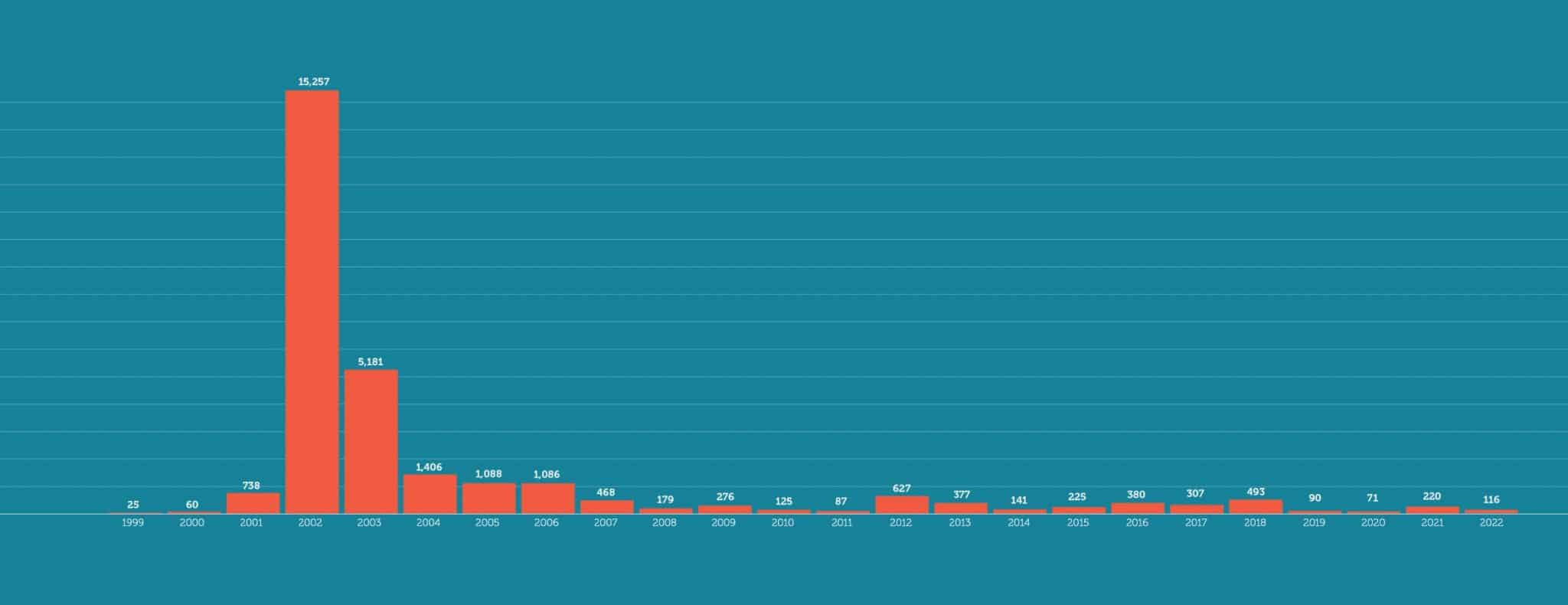
Number of Reported U.S. Equine WNV Cases by Year from CDC data
WNV: WHERE WE STAND TODAY
Today, according to CDC data, 48 states and the District of Columbia have reported cases of WNV in humans, horses, mosquitoes, or other animals (all except Hawaii and Alaska). State veterinarians reported only 116 equine WNV cases in 2022, down from a high of more than 15,000 two decades prior in 2002. However, experts agree that this statistic doesn’t accurately reflect the actual number of cases.
“West Nile virus in horses is largely under-reported,” explains Dr. Angela M. Pelzel-McCluskey of the USDA. “The cases reported through ArboNET (a CDC arbovirus surveillance site), and subsequently on the APHIS website, are confirmed at a laboratory by diagnostic testing and officially reported by a veterinarian to state authorities,” she said.
This means that for cases to be included in the “official” number of infections, a horse owner has to pay for testing and veterinarian has to take a diagnostic sample, submit it to the laboratory, and report findings to the state if the test comes back positive.
“Not all owners can, will, or want to test their horses, and there are also many veterinarians who may not specifically recommend WNV testing to a horse owner to confirm a case,” Pelzel-McCluskey says. “For example, if the horse is not vaccinated and has clinical signs consistent with WNV infection, the veterinarian and/or owner may not think it necessary to get the diagnostics done to ‘prove’ it.”

Angela Pelzel-McCluskey,
DVM, MS
is an equine epidemiologist with USDA-APHIS-Veterinary Services.
CURRENT VACCINE OPTIONS
Veterinarians agree that vaccination is the best way to protect your horse from WNV, which is why it is included in the AAEP’s core vaccination schedule as a vaccine horses should receive at least once a year.
Dr. Maureen Long of University of Florida has treated horses since WNV’s early introduction into the United States and is a strong advocate for vaccination.

Maureen Long, DVM, PhD, MS, Dipl, ACVIM
is an associate professor of infectious disease and pathology at the University of Florida’s College of Veterinary Medicine, in Gainesville
“One in 11 horses exposed to WNV via mosquito bite will develop neurological disease,” Long points out. “About three of every 10 horses that become neurological will die from the disease. One of the remaining three will not recover completely. Lower numbers of horses than humans become sick each year from WNV because of the success of vaccination. WNV is entirely preventable in the fully and repeatedly vaccinated horse.”
Additionally, veterinarians recommend that owners waffling about vaccinating their horses against WNV should consider that:
- The WNV vaccine, depending on the chosen product, can cost as little as $25;
- 10-39% of unvaccinated horses inoculated with WNV via a mosquito show signs of infection;
- Vaccination provides horses nearly 100% protection against disease caused by WNV; and
- The cost of supportive care for an infected horse can exceed $10,000 (cost varies based on severity of disease and location or clinic).
Veterinarians play a key role in informing their clients about the importance of vaccinating horses against WNV regularly, says Traub-Dargatz, and especially when mosquito numbers are high and WNV is detected in mosquito pools. (Horse owners can stay abreast of WNV surveillance numbers on USDA APHIS’ Equine Arbovirus Dashboard.)
“I would recommend that horse owners work with their veterinarian to determine what is the best vaccination schedule for their horse since the veterinarian will be familiar with the current risk level for West Nile virus in their area,” says Dr. Kevin Hankins of Zoetis, emphasizing that the number of reported cases could be far lower than reality because numerous states no longer report or require reporting WNV to officials.
“Increases in WNV cases can happen rapidly depending on the environmental conditions and number of infected mosquito pools in an area, so having your horse vaccinated in advance of any increase in exposure to infected mosquitoes is the best thing the horse owner can do to protect their horse from a potentially deadly disease,” he adds. “Horse owners want to make sure that their horse has established a proper immune response to WNV well in advance of any potential increase in exposure.”
Killed-virus
An inactivated vaccine (or killed vaccine) consisting of virus particles grown in culture and then killed. These viruses are grown under controlled conditions and are rendered noninfectious.
Recombinant Vaccine
A recombinant vaccine is a vaccine produced through recombinant DNA technology, which involves inserting the DNA encoding an antigen that stimulates an immune response.
Chimera Vaccine
A chimera vaccine is created by genetically splicing two viruses together, producing a “chimeric” structure.

Kevin Hankins, DVM,
MBA,
is a managing equine veterinarian with Zoetis.
The U.S. market currently offers four WNV vaccines: two killed-virus, one canarypox recombinant, and one chimera.
The AAEP describes the vaccines and their respective labels in its WNV vaccination guidelines:
“Inactivated whole virus vaccines with an adjuvant. Label instructions call for a primary vaccination series of two intramuscular injections administered three to six weeks apart followed by a 12-month revaccination interval. These products are labeled as an aid in prevention of viremia or as an aid in prevention of viremia and mortality and an aid in reduction of severity of clinical disease.
“Recombinant canarypox vaccine with protective antigens expressed in a vaccine strain canary pox vector which does not replicate in the horse. The vaccine contains an adjuvant. Label instructions call for a primary vaccination series of two intramuscular injections administered four to six weeks apart followed by a 12-month revaccination interval. The product is labeled as an aid in prevention of disease, viremia, and encephalitis.
“Inactivated flavivirus chimera vaccine with protective antigens expressed in a vaccine strain yellow fever virus vector and contains an adjuvant. Label instructions call for a primary vaccination series of two intramuscular injections administered three to four weeks apart followed by a 12-month revaccination interval. This product is labeled as an aid in reduction of disease, encephalitis, and viremia.
“All of the current WN vaccine products carry one-year duration of immunity, with challenge, consistent with their respective label claims.”
Long and colleagues at University of Florida conducted one head-to-head trial using three of the four (one each of killed virus, canarypox recombinant, and chimera). They administered one of the three vaccines according to the manufacturer’s directions, with the exception of control horses that received only a diluent.
The researchers “challenged” the horses with WNV using an intrathecal method (i.e., directly into the cerebrospinal fluid) 28 days later. All six unvaccinated horses developed WNV, demonstrating the severity of the challenge method.
Of the vaccinated horses, “Regardless of what vaccine was administered, survivorship was 100%,” Long says.
Still, vaccines can’t exert their protective effects if owners don’t prioritize vaccination, Hankins notes.
“I have seen and read of a number of increased cases of WNV in regional areas across the United States where the vaccination compliance rate has decreased,” he says. “With a disease like WNV that has over a 30% mortality rate in horses, and knowing that the virus is endemic in the United States, vaccination for West Nile (as well as the other diseases in AAEP’s core vaccine list ) would be highly recommended to continue to protect horses.”
For vaccination schedules for different horse demographics with different vaccination histories, Long says to follow the AAEP’s WNV vaccination guidelines.
WHY SOME HORSES MIGHT GET WNV, AND SOME DON'T
In North America researchers have identified equine risk factors for developing WNV encephalitis, but the overall significance of those remains unclear. Dr. Hugh Townsend and Dr. Tasha Epp of The University of Saskatchewan’s College of Veterinary Medicine published risk factor data from their research.
“We assessed factors that contributed to death in 133 clinical cases; namely, week of onset of symptoms, gender, and coat color after accounting for regional differences,” says Epp. “The survival rate of horses with clinical signs presenting earlier in the season (i.e., Weeks 31-33 of the calendar year) was higher compared to horses with signs that developed later in the season (i.e., Weeks 36-38). Stallions were more at risk of death than either mares or geldings, and light-colored horses were more at risk of death, which was something we could not explain.”
Long notes that the latest research, however, shows no consistent effect of gender.
Other studies conducted in the United States yielded different results regarding risk factors. In one 2004 study researchers found that factors associated with mortality from clinical disease included lack of vaccination, advanced age, inability to rise as a clinical sign, early season onset of clinical disease (the timing differs, depending on when the WNV season starts in different areas of North America), sex, and breed.
“We did not find any of the same factors in our study,” reports Epp, and Long notes that research in recent years has shown there is no age association with mortality from WNV clinical disease.
In the southern states, such as Florida, mosquitoes and the possibility of them carrying WNV is a year-round challenge. No matter the location, using barn-safe electric fans, housing horses in barns, and blanketing reportedly decrease the risk of WNV infection, presumably because those strategies decrease possible exposure to infected mosquitoes.
And, again, vaccination is key. In these regions with extended mosquito seasons, veterinarians might booster more than once annually.
“Administering a WNV booster would be recommended for those horses that are at risk of infection for several reasons,” says Hankins. “Risk factors can include prolonged mosquito exposure due to environmental conditions, older horses, immune-compromised horses (such as those with metabolic syndrome), horses that travel, or horses with an unknown vaccination history.
“It is best for horse owners to work with their veterinarian to determine if their horse is at risk for increased exposure or susceptibility to WNV to determine if their horse would benefit from a booster vaccination,” he adds.

Hugh Townsend, DVM, MSc,
is currently professor emeritus at the University of Saskatchewan, in Canada. At the onset of the WNV outbreak in North America, he was teaching veterinary internal medicine in the Department of Large Animal Clinical Sciences, Western College of Veterinary Medicine; an adjunct professor in the Department of Community Health and Epidemiology, Royal University Hospital, University of Saskatchewan, teaching analytic epidemiology; and a research scientist at the University of Saskatchewan’s Vaccine and Infectious Disease Organization.
Tasha Epp, DVM, PhD,
is a professor of epidemiology/zoonosis in Large Animal Clinical Sciences at the University of Saskatchewan’s Western College of Veterinary Medicine.
WNV'S FUTURE
West Nile virus is a permanent resident in North America. And despite many scientists initially attempting to determine where the virus would go and how it would behave in North America, they almost unanimously agree that it was, and continues to be, impossible to predict.
Researchers say they used to wonder what would happen when the virus changes or mutates. Examples of other organisms that have changed include methicillin-resistant Staphylococcus aureus and equine herpesvirus-1. In the latter case, one single change of the virus at the DNA level, called a point mutation, resulted in a virus that caused neurologic disease rather than the more typical respiratory disease.
But, Long reports, multiple new strains of WNV have arisen worldwide in the intervening years, and “all studies to date show that the vaccines are efficacious against the new strains.”
Multiple new strains of WNV have arisen worldwide in the intervening years, and research results have shown vaccines are efficacious against them.
Komar says vector-borne viruses undergo evolutionary change slower than other viruses, because they replicate in both vertebrate and invertebrate hosts. This means that a mutation that provides an evolutionary advantage for virus replication in mosquitoes might be disadvantageous for replication in vertebrate hosts.
“It is unlikely that the changing genotype of WNV in the U.S. will impact horse or human illness patterns,” Komar says. “However, it is not impossible, and another virus with a similar ecology has done just that. The WEE virus rarely causes disease in either people or horses in the 21st century, but it was a major cause of equine disease in the last century.”
West Nile Virus’ Travel Throughout the U.S.
The West Nile virus (WNV) had its passport stamped by the United States in 1999, entering the country by way of New York. The jet-setting virus promptly and aggressively spread its wings both to the south and west, ultimately infecting horses in almost every state by 2002-2003.
Despite the availability of a vaccine, one that was rapidly rolled out by September 2001, hundreds of horses continue to be infected annually throughout the country.
“In addition to the loss of tens of thousands of horse lives, this virus continues to cause economic hardship through the loss of use of horses that have not completely recovered from previous infection and through the cost of at least annual or more frequent vaccination for WNV that all horse owners must endure now to keep their horses safe,” says Dr. Angela Pelzel-McCluskey, the equine epidemiologist of the USDA-Animal and Plant Health Inspection Service (APHIS), located in Fort Collins, Colorado.
To better understand WNV’s current and future effects on the equine industry, including private horse owners, Pelzel-McCluskey and a team of researchers conducted a comprehensive analysis of the “spatiotemporal” patterns of the virus (Humphreys et al., 2021).
“Given the ‘extraordinary rapidity’ with which the WNV can move across large distances, the goal of that study was to use advanced epidemiologic techniques to predict when and where the virus is likely to spread throughout the contiguous United States,” says Pelzel-McCluskey. “This information, referred to as spatiotemporal, is anticipated to improve disease surveillance to better identify at-risk horses. In turn, horses at higher risk of infection can be prioritized for vaccination programs and those geographical areas subject to heightened virus surveillance and monitoring.”
Briefly, the research team employed disease biogeography to analyze the horse hosts, mosquito vectors, avian reservoir hosts, and climatic/environmental factors. Their key findings included:
- The risk of WNV disease in horses was not uniform across the United States or consistent throughout the year, as was expected.
- July to October was the highest risk period nationwide.
The study results revealed several high-risk clusters, including Central Pennsylvania and Lower Louisiana.
“These are along the migratory flight paths of birds, linking the Gulf Coast to the northeastern United States,” says Pelzel-McCluskey.
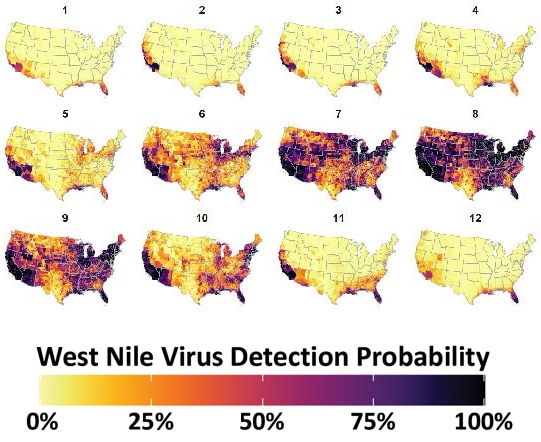
WNV detection probability. WNV surveillance covariate estimated from virus detections reported to the CDC. Mapped counties are color coded according to legend at bottom to indicate WNV detection probability (converted to percent chance). Darker tones indicate an elevated chance of virus detection whereas lighter tones represent a lesser chance of detection. Covariate construction is detailed in Section 2.2 of the study. Figure used under the creative commons license.
Using spatiotemporal modeling, the research team found that WNV shifts from south to north during the onset of summer and identified a north-to-south recession associated with the start of winter. For the duration of the summer (June to September), most of the United States has WNV coverage.
Thus, during the coldest months (November to April) virus occurrence is limited to the southern and coastal portions of the United States.
“The thermal buffering from the Atlantic and Pacific oceans moderates low-temperature extremes, likely providing winter refuge for birds that might serve as reservoirs for the virus,” Pelzel-McCluskey explains. “But it is also possible that managed water and sewer systems in some coastal locations may facilitate mosquito overwintering.”
Increased precipitation corresponded to elevated risk of WNV disease in horses in some regions of the United States (e.g., northernmost western states, mid-Atlantic states). But, in others such as west of the Mississippi, the Southeast, and California, increased precipitation was associated with a decreased risk of disease.
“A similar inconsistent finding was appreciated with temperature,” Pelzel-McCluskey adds. “In some regions of the United States, high temperatures increased West Nile disease risk whereas in other regions, there was a decreased risk of disease with elevated temperatures.”
Interestingly, she says, drought had a “dynamic” relationship with WNV disease. Specifically, nondrought conditions were associated with an increased risk of disease while abnormal dryness had a suppressive effect. Exceptional drought also had a negative influence on disease.
“Moderate drought conditions may reduce host (horse and bird) and vector (mosquito) water access, resulting in less vector abundance combined with fewer avian hosts present to provide the virus in that area,” says Pelzel-McCluskey. “But during times of extreme drought, water becomes so rare that all the hosts and vectors collide at those available water sources, increasing risk of disease.”
In conclusion, the spatiotemporal identification of WNV allows a more comprehensive understanding of where in the United States and at what times of year specific equine populations are more at risk of infection. Veterinarians should target these populations for vaccinations/boosters and vector control strategies.
“While this research is extremely valuable, more research looking at the impacts of climatic and ecological factors on WNV risk is needed to evaluate the effects that changing climate may have on WNV and other vector-borne diseases in the future,” says Pelzel-McCluskey.
*Humphreys JM, Pelzel-McCluskey AM, Cohnstaedt LW, McGregor BL, Hanley KA, Hudson AR, Young KI, Peck D, Rodriguez LL, Peters DPC. Integrating Spatiotemporal Epidemiology, Eco-Phylogenetics, and Distributional Ecology to Assess West Nile Disease Risk in Horses. Viruses. 2021; 13(9):1811. https://doi.org/10.3390/v13091811 Copyright 2021 by the authors, Licensee MDPI, Basel, Switzerland.
WHAT has WNV Taught US?
Almost 24 years ago, WNV caught human and horse health authorities by surprise when it entered the United States. Representatives from all corners of the industry rallied, educating horse owners on mosquito reduction techniques and creating an equine vaccine to save horses’ lives. Today, even more than then, the world is one community due to growing international equine movement and competition, and horses in North America simply make up a herd on a global scale, says Traub-Dargatz. “There will be another (new disease outbreak), it’s just a matter of time.” But, due in part to lessons learned during WNV’s emergence, we are better prepared for the “next West Nile virus,” whatever it may be. In the meantime, veterinarians agree, protect your horses from the real and known threat of WNV.
“From a disease prevention perspective, horse owners are extremely lucky that there are WNV vaccines that have shown to be very effective in preventing the disease in properly vaccinated horses,” says Hankins. “As the number of horses that are vaccinated has increased, we have seen a dramatic decrease in the number of cases of the virus, and the cases we do see are in nonvaccinated or improperly vaccinated horses. However, it is important horses receive yearly vaccination for WNV and the other core equine diseases (rabies, tetanus, EEE, WEE) so that we continue to see lower numbers of cases of these potentially deadly diseases.”
CREDITS:

Stacey Oke, DVM, MSc,
Editorial Director: Stephanie L. Church
Designer: Kimberly Reeves
Former Digital Managing Editor: Michelle Anderson
Editorial Team: Alexandra Beckstett, Haylie Kerstetter
Art Director and Graphics: Claudia Summers
Web Producer: Jennifer Whittle
Publisher: Marla Bickel





