Bisphosphonates for Horses: A Review
- Topics: Article, Horse Care, Medications, Welfare and Industry
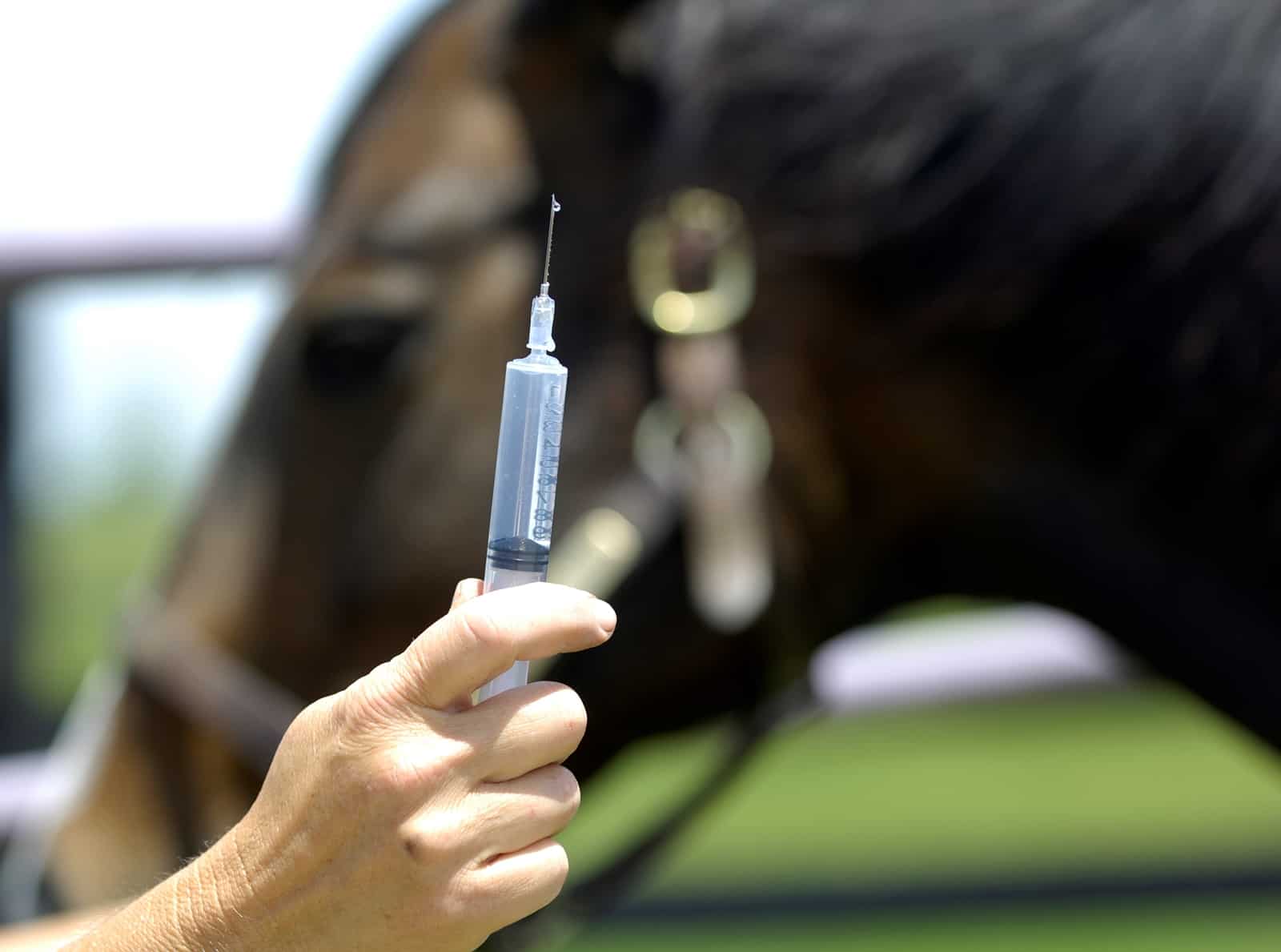
For years, equine veterinarians relied on drugs, corrective shoeing, and surgery to help horses suffering from navicular syndrome to minimize discomfort and disease progression.
Also known as podotrochlosis, navicular syndrome is the degeneration of the navicular bone nestled near the coffin bone near the rear of the horse’s foot, along with its associated structures. Management of this disease changed significantly in 2014 when the U.S. Food and Drug Administration approved bisphosphonates for treatment. Despite the availability of this newer treatment option, success rates—in terms of improvement in lameness grade—hovered around the 67% mark, leaving many horses in continued pain.
Recently, Texas A&M researchers reviewed the current body of knowledge surrounding bisphosphonates and their use in horses. Their goal, they wrote, was to “discuss the current understanding of the strengths and weaknesses of BPs in equine veterinary medicine and highlight the future utility of these potentially highly beneficial drugs
Create a free account with TheHorse.com to view this content.
TheHorse.com is home to thousands of free articles about horse health care. In order to access some of our exclusive free content, you must be signed into TheHorse.com.
Start your free account today!
Already have an account?
and continue reading.

Written by:
Stacey Oke, DVM, MSc
Related Articles
Stay on top of the most recent Horse Health news with











