Awesome Antioxidants and How They Help Horses
- Topics: Horse Care, Nutrition, Sports Medicine, Sports Nutrition

These microscopic compounds help abolish damaging free radicals within horses
Oxygen, while necessary for fueling life, possesses damaging doppelgängers called reactive oxygen species. These highly charged, reactive forms of oxygen, known as free radicals, contribute to mass destruction at a microscopic level. Luckily, the body comes equipped to handle free radicals using vitamins, proteins, enzymes, and minerals that exert antioxidant activities. These antioxidants help cells, tissues, and organs weather harmful free radical damage.
To better understand the antioxidant and free radical “dance,” picture a team roping event at a rodeo. The steers are the free radicals while the horse and rider pairs are the antioxidants. The steers wait in a holding pen, “metabolizing,” when suddenly one is released, highly reactive and careening across the ring. The header, hot on the steer’s trail, easily subdues it with the help of the heeler and minimizes collateral damage and bystander injury inflicted by the errant free radical.
Where do free radicals come from, and how do antioxidants regain control of these wayward molecules? We’ll answer those questions and more, focusing on your horse’s main antioxidant systems.
Why Horses Need Antioxidants
All metabolic processes, even the normal, everyday chemical reactions that take place in the body to sustain life, generate free radicals. Take, for example, the breakdown of sugar molecules (glucose) in muscle cells to drive muscle contraction.
“Sugar molecules from the horse’s feed are absorbed from the small intestine and circulate throughout the body in the bloodstream,” says Carey Williams, PhD, an equine extension specialist at Rutgers University, in New Brunswick, New Jersey, who has studied exercising horses and the effects of oxidation and antioxidant supplementation for 20 years. “Muscle cells take up and metabolize those sugar molecules to produce energy, water, and carbon dioxide. Oxygen plays a key role in breaking down or ‘oxidizing’ sugar into these base components.
In this representation of how sugar is oxidized, the reaction looks perfect: Six oxygen molecules react with one sugar molecule to produce six carbon dioxide and water molecules. But oxygenation is far from perfect.

“In real life, oxidation is an imperfect process that often results in certain oxygen molecules escaping from this chain of events,” says Williams.
A more realistic image of glucose metabolism looks like this:
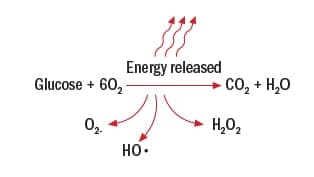
Here, O2-, HO•, and H2O2 are types of free radicals or reactive oxygen species— superoxide, hydroxyl , and hydrogen peroxide, respectively.
“These free radicals are reactive because they each have unpaired electrons,” says Williams. “These lonely electrons give the molecule a negative electric charge that makes them seek out positively charged molecules to become neutral. In fact, the negatively charged free radicals are so driven to become neutral that they blindly blunder around the muscle cell, smashing into other molecules, enzymes, cell membranes, and even DNA. When these reactive oxygen species overwhelm the system, we term that ‘oxidative stress.’ ”
Free Radicals Everywhere
A common source of free radical generation is muscle metabolism that’s producing energy for exercise.
“All cells need to produce energy, so all cells in the body produce free radicals,” says Williams.
Inflammation, immune cell activation, infection, intense or endurance exercise, UV radiation exposure, cancer, and aging can generate free radicals, as well. Exposure to certain compounds in the environment can also produce free radicals. Examples include chemicals ingested or inhaled; think water and air pollution, some drugs (e.g., antibiotics such as cyclosporine and gentamicin), secondhand cigarette smoke, and heavy metals (lead, cadmium, mercury).
The Effects of Free Radical Damage
As Williams described, oxidative damage occurs when excessive amounts of free radicals get produced. Those free radicals bounce around the cells, causing= damage to everything in their wake—cell membranes, structural proteins, and enzymes (proteins that act as biological catalysts), as well as DNA.
Let’s look at cell membrane damage in more detail. Cell membranes are made up of row upon row of long, slender fat molecules stacked side by side like books on a library shelf. These fatty membranes form barriers between cells and the extracellular environment and even between different organelles within a cell. Examples of organelles include the mitochondria, which produce the bulk of the cell’s energy, and the nucleus, which contains the majority of the cell’s DNA.
These cell membranes control the flow of electrolytes (sodium, potassium chloride), sugars, and proteins, signaling molecules across the membrane to store them or drive physiological processes such as nerve impulse transmission and muscle contraction.
“When free radicals strike these membranes, the individual fatty acid molecules become damaged, contorted, and fail to maintain membrane integrity,” Williams says. “This means that the electrolytes, proteins, etc., that are supposed to be kept on one side of the membrane or the other can freely pass through the membrane, interrupting vital cellular processes. They can even totally destroy the cell.”
A single free radical damaging a single fatty acid molecule can start this lipid peroxidation (oxidative degradation) process. Because the lipid molecules are packed together tightly, once one fatty acid molecule becomes oxidized or “reactive,” a chain reaction of oxidation starts damaging each fatty acid—as if pushing those library books off the shelf one after the other in quick succession.
When free radicals strike proteins and DNA, these molecules can suffer structural and functional damage.
Proteins acting as enzymes that sustain this damage “can no longer catalyze chemical reactions, such as those needed to produce energy from sugar,” says Williams.
Free radical damage causes point mutations in DNA that lead to the production of abnormal proteins and enzymes. Such mutations occur when one or more DNA sections (called “bases”) that are supposed to code for one type of amino acid used to build proteins become damaged. The DNA puts a different amino acid into the protein instead, causing it to malfunction.
Natural Antioxidant Systems in the Horse’s Body
Because oxidation is an essential bodily process, the horse comes with built-in antioxidant systems that utilize vitamin E, vitamin C, glutathione, and selenium, among other antioxidants.
Vitamin E is the king of antioxidants. This fat-soluble molecule slips between individual fatty acids that make up the bulk of cell membranes. Vitamin E stops the chain reaction of lipid peroxidation by oxidizing into a form that is not reactive like the oxidized fatty acid molecules of the membrane.

Vitamin E plays a very important role in the health and stabilization of many body systems. The immune system, for example, has cells containing very high concentrations of polyunsaturated fatty acids easily oxidized by free radicals.
In one study researchers found that horses fed diets low in vitamin E but subsequently supplemented with vitamin E (either with or without selenium) mounted a better immune response to vaccination than did unsupplemented horses (Petersson et al., 2010). They noted increased IgG (a first-line infection-fighting protein that circulates in the bloodstream) levels only in horses offered supplemental vitamin E.
A similar phenomenon happens with oxidating muscle cells, says Williams. “Research shows that if intensely exercising horses are given antioxidants like vitamin E and/or vitamin C, the muscle cell membranes are protected, creating less muscle cell damage during exercise.
“We can detect this membrane leakage by measuring the amount of muscle enzymes that leak out of the cell into the bloodstream,” she adds. “Normally, there are low concentrations of those enzymes in the blood, as the concentrations increase as the membranes become leaky.”
Vitamin C, a water-soluble antioxidant, floats fairly freely within the cell, squelching errant free radicals bouncing around. Vitamin C also moonlights as a vitamin E regenerator to deoxidize vitamin E molecules within cell membranes so they can continue to stop the lipid peroxidation chain reaction.
“Vitamin C and vitamin E work together in perfect combination to fight free radicals and regenerate the antioxidants so they can continue the cycle over and over again,” says Williams. “When antioxidants cooperate in this manner, we call it potentiation.”
Glutathione is an antioxidant that wears many hats. Similar to vitamin C, it can function as an antioxidant on its own accord (while vitamin E essentially acts as an oxidation barrier). Glutathione also plays a pivotal role in the functioning of the antioxidant enzymes glutathione peroxidase and reductase. In addition, glutathione plays an important role in regenerating vitamin C and E after they have been oxidized—like a boxing coach who squirts water in the boxer’s mouth, pats him on the shoulder, and sends him back in for another round. Some of Williams’ research has shown that the boxers sometime return the favor; supplementing horses with antioxidants such as vitamin E and vitamin C also increases glutathione concentrations.
Selenium helps glutathione peroxidase function. In addition, this water-soluble mineral pairs with vitamin E to form an antioxidant system to be reckoned with.
Other antioxidants include a variety of enzymes within cells that serve as radical squelchers by donating electrons. Examples include superoxide dismutase and coenzyme Q10. Minerals besides selenium can also play a role in oxidation-reduction reactions, acting as cofactors (nonprotein chemicals that assist with biological reactions) to certain enzymes. The antioxidant enzyme catalase, for example, cannot function properly without iron.
“And even though we list all these antioxidants individually here, it is important to appreciate that all antioxidants work better in combination,” Williams says.

Oxidative Stress During Exercise
Free radical production can’t be stopped, only controlled by the body’s natural antioxidant systems and exogenous (supplemental) antioxidants. Oxidative stress occurs in many endurance horses and other mounts performing intense exercise, such as three-day eventers.
Williams has found in various studies that horses completing endurance-type exercise (i.e., 80-mile races) experience lipid peroxide increases in the blood indicative of an increase in reactive oxygen species. In addition, muscle enzymes leak out of the muscle cells into the bloodstream, indicating that the horse might be experiencing oxidative stress.
“My research shows that supplementing horses undergoing this level of exercise with 5,000 IU vitamin E per day could decrease the membrane permeability of these muscle cells by stabilizing the membranes and presumably minimize oxidative stress,” says Williams.
Therefore, she says, it’s a good idea to make sure your intensely working horse either has access to green pasture to meet his vitamin E requirement or consumes a diet fortified or supplemented with vitamin E or an antioxidant blend.
Williams says this level of vitamin E supplementation might also increase the concentration of other antioxidants available to help combat oxidative stress.
Dr. Carey Williams
Free Radical Damage and Disease
Oxidative stress can cause or hasten various disease processes. Human diseases scientists believe to be related to oxidative stress include:
- Atherosclerosis and other cardiovascular diseases;
- Asthma;
- Arthritis;
- Memory loss, behavior changes, depression, degenerative changes;
- Renal (kidney) failure;
- Cancer;
- Cataracts and retinal diseases; and
- Inflammation and infection.
“Diseases related to oxidative stress in horses haven’t been studied as widely as in humans,” says Williams. “Some evidence exists that oxidative stress has some effect on chronic obstructive pulmonary disease (severe asthma) in horses, as well as cataract development in horses with low vitamin A levels, which also has antioxidant properties. I would suspect that the development of arthritis is linked to oxidative stress in horses like it is in humans.”
In a 2020 study researchers attempted to address the role of oxidative stress in aging and pituitary pars intermedia dysfunction (PPID, equine Cushing’s disease), an age-related neurodegenerative disease in horses. Lead author Agnieszka Zak, PhD, of the Wrocław University of Environmental and Life Sciences, in Poland, relayed important concepts regarding the toll free radicals take on an animal’s body.
“A weakening of the body’s antioxidant function may occur as an animal ages,” she explains. “This does not say that a causal relationship exists, just that there is an association between the two events.”
For example, some study results show the activity level of the antioxidant enzyme superoxide dismutase and total antioxidant capacity decrease in horses with advancing age. Researchers on other studies have reported no such occurrence.
Zak and colleagues wanted to find out if oxidative stress is connected to advancing age and PPID development caused by neurodegeneration of dopaminergic neurons in the hypothalamus, which can lead to cellular hyperplasia (increase in numbers) and micro- and macroadenoma (noncancerous tumors) formation in the pars intermedia (part of the pituitary). Zak says these can stimulate the overproduction of hormones such as adrenocorticotropin hormone, increased levels of which are responsible for the clinical signs of PPID. They theorized that PPID and advanced age would negatively affect antioxidative stress systems and reduce horses’ ability to combat oxidative stress.
She and her team did not, however, find any associations between PPID—or even insulin dysregulation—and oxidative stress measured in serum in older horses.
“This lack of association may suggest that aged horses can cope with oxidative stress, but this topic certainly requires more research,” Zak says.
Take-Home Message
Providing horses with the tools to squelch free radicals starts with a balanced diet containing the necessary vitamins and minerals. Adding more vitamin E and other antioxidants to their base diets might improve their ability to fight oxidative stress, particularly when performing intense levels of work.
“But antioxidants, like all other vitamins and minerals, must be provided in a balanced fashion,” says Williams. “Oversupplementation is overkill in most cases, and remember some oxidation is necessary for helping the immune system fight off infections. Like many things in nutrition, it is best to find a balance between the good and the bad.”

Related Articles
Stay on top of the most recent Horse Health news with

















