Equine Stem Cells Rein in Bacteria
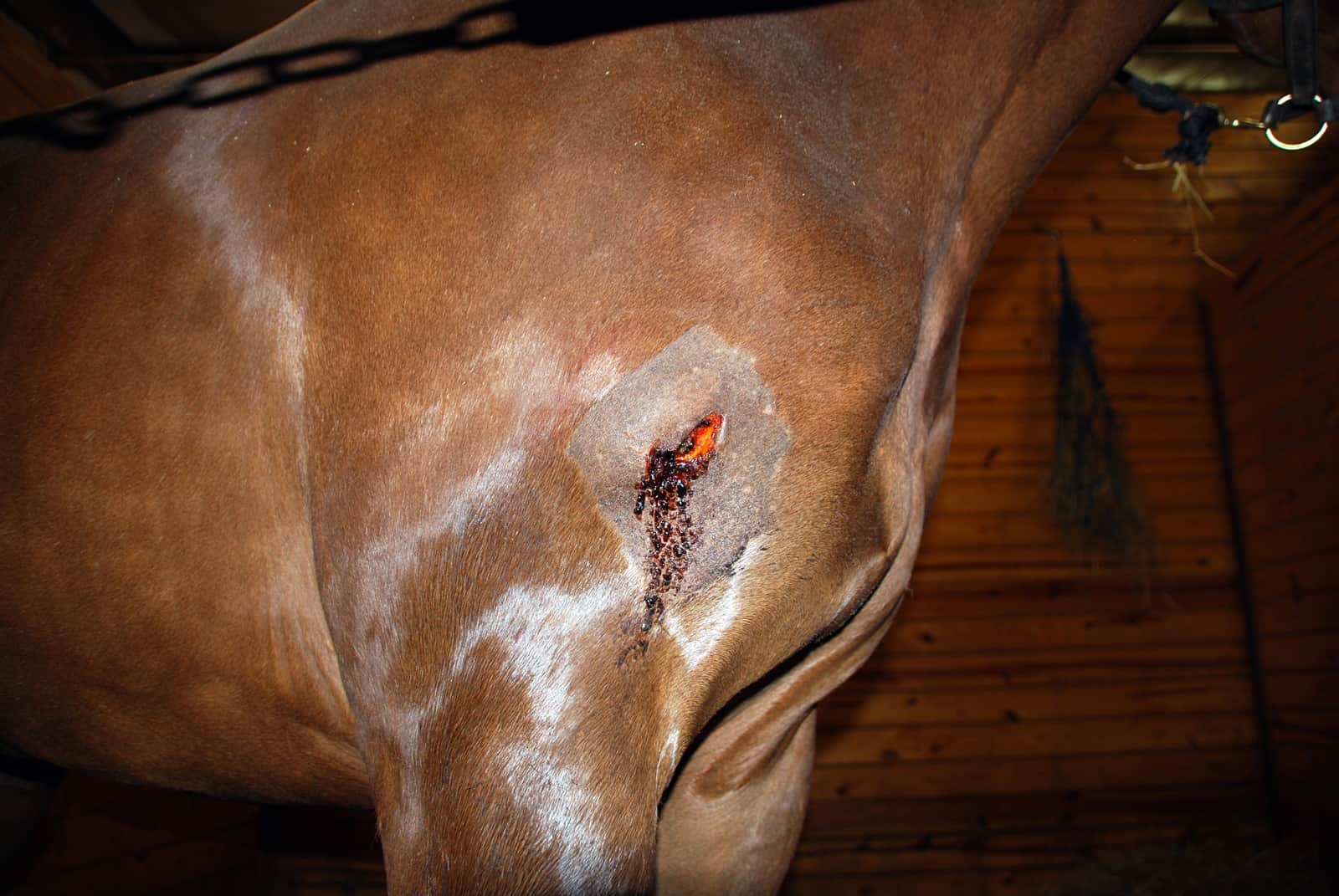
Rebecca Harman, a research support specialist and PhD student, and colleagues in the Van de Walle Lab at Cornell’s College of Veterinary Medicine, in Ithaca, New York, have found that factors secreted by adult stem cells, also known as mesenchymal stromal cells (MSCs), appear to be able to fight bacteria commonly found in skin wounds.
Bacteria often complicate the treatment of chronic skin wounds in humans, driving a need for new therapies that reduce bacteria in wounds. Although previous research has explored the therapeutic value of MSC in wound healing, few studies examined the potential for MSCs to inhibit bacterial growth. Harman and the Van de Walle Lab are examining the antibacterial properties of equine MSC secreted factors, like antimicrobial peptides, to develop therapies for horses and to serve as a model for human studies.
“This equine skin wound healing model offers a readily translatable example for MSC therapies in humans,” said Harman. “Although mice are smaller and less expensive model organisms, the horse is more physiologically relevant when it comes to human skin wound healing
Create a free account with TheHorse.com to view this content.
TheHorse.com is home to thousands of free articles about horse health care. In order to access some of our exclusive free content, you must be signed into TheHorse.com.
Start your free account today!
Already have an account?
and continue reading.
Written by:
Edited Press Release
Related Articles
Stay on top of the most recent Horse Health news with















